Edited by Gregory2455, December 27 2014 - 2:03 PM.
- Formiculture.com
- Forums
- Gallery
- Members
- Member Map
- Chat

Edited by Gregory2455, December 27 2014 - 2:03 PM.
I setup a couple tests to see if I can solve this erosion problem.
I took a piece of a synthetic chamois that seems to be made from something very similar to a PVA sponge, and laid it down on the floor of the container, and then poured new Hydrostone over that. I just want to see what this will do to the path of the water. I ultimately have an idea that could possibly help, I'm just not sure because I'm not an expert in physics. I want to test it out each step of the way, just to get an idea of how each part of this idea affects the water flow and erosion. I filled the container with peat moss again like I did the first time I discovered this problem while testing my cricket bin idea. This moves a large amount of water through the Hydrostone, speeding up the process. It took less than eight hours to bore a pin hole right through 1/4 inches of Hydrostone last time, so this should give me a good comparison.
I also put a 3mm layer of Hydrostone in the bottom of one of the tanks as discussed here (http://www.formicult...-7-2014/?p=9783). This should allow the Hydrostone in the tank to change the pH of the water so it will be the same as the Hydrostone it's passing through. If this is mainly caused by the pH of the water, then this might help. Once the other test is finished I'll give this one a try. This should give it plenty time to change the pH of the water.
I have also ordered a bisque tile, which is an unglazed ceramic tile. These should absorb water and act a lot like Hydrostone. Since these are not cement based, and very hard, I suspect they will not have this erosion problem. I'll be test this out once it arrives.

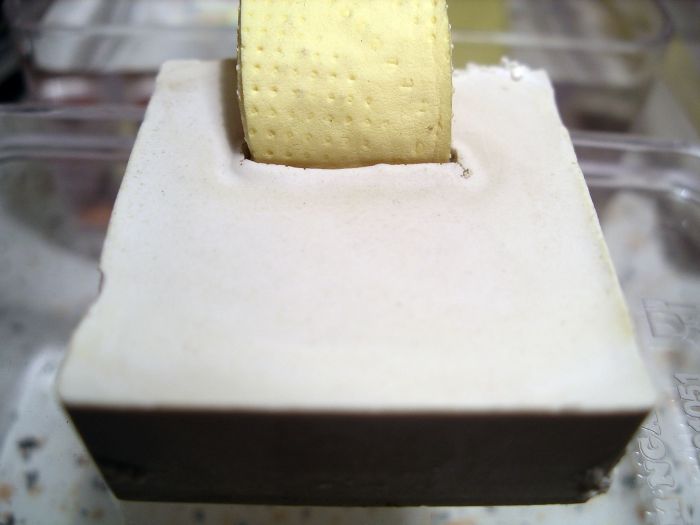




Those white deposits are what are called "sweat". That is what I was referring to when I mentioned hydrostone sweat. It has been recorded in a number of journals, although the nests were setup a lot like my mine (flat with a water chamber on top).
Did the bubbles develop within the first 1-3 hours? If so, then it was just from the water, I see it all the time when I pour a glass of water
If it occurred after 5 hours, then it is likely something else - maybe the hydrostone releasing very tiny bubbles of air from deeper in hydrostone?
"Always do right. This will gratify some people, and astound the rest." -- Samuel Clemens
The Hydrostone does release small bubbles as water soaks into it, but these appear on every surface in the containers that don't even have Hydrostone in them.
What it looks like to me is that some chemical reaction is occurring and forming precipitate deposits on the hydrostone. I'm no industrial chemist so I have no idea what, but that may help you focus your search for an answer. The bubbles could be a gaseous product of whatever reaction is occurring. If you have access to a highly sensitive digital balance, you should mass the hydrostone blocks before and after to see if those bubbles actually are a product of some reaction.
Edited by kellakk, January 22 2015 - 1:12 PM.
Current Species:
Camponotus fragilis
Novomessor cockerelli
Pogonomyrmex montanus
Pogonomyrmex rugosus
Manica bradleyi
I recently read the MSDS of Hydrostone and to my surprise, it's actually made up of more than 95 percent Plaster of Paris (Calcium Sulfate Hemihydrate) (CaSO4•½H2O)
You can see the MSDS for yourself here (http://www.usg.com/c...en-52140052.pdf).
I posted this problem on a Physics/Chemistry forum (https://www.physicsf...-cement.793777/), and at the time of this post have been told this is simply due to the Plaster of Paris dissolving in the water, and that possibly saturating the water may solve the problem.
I also found this (http://www.howtoclea...is-from-cement/), where they claim that the way to dissolve Plaster of Paris is to use something acidic. According to one of the commenters, this information is incorrect and only copied from another incorrect source. He says he experimented and could not get anything acidic to dissolve the plaster, but instead dissolved it with something at the other end of the pH scale. He also says this correct information is impossible to find on the internet. I even found what I suspect was a post of his over on the very Physics forum I posted my question on where he actually corrected all of them on the answer, with not one rebuttal. Unfortunately, this doesn't leave me too confident in the help I'm going to receive over there...
In other words, you opened a can o' worms... ![]()
I recently read the MSDS of Hydrostone and to my surprise, it's actually made up of more than 95 percent Plaster of Paris (Calcium Sulfate Hemihydrate) (CaSO4•½H2O)
You can see the MSDS for yourself here (http://www.usg.com/c...en-52140052.pdf).
I posted this problem on a Physics/Chemistry forum (https://www.physicsf...-cement.793777/), and at the time of this post have been told this is simply due to the Plaster of Paris dissolving in the water, and that possibly saturating the water may solve the problem.
I also found this (http://www.howtoclea...is-from-cement/), where they claim that the way to dissolve Plaster of Paris is to use something acidic. According to one of the commenters, this information is incorrect and only copied from another incorrect source. He says he experimented and could not get anything acidic to dissolve the plaster, but instead dissolved it with something at the other end of the pH scale. He also says this correct information is impossible to find on the internet. I even found what I suspect was a post of his over on the very Physics forum I posted my question on where he actually corrected all of them on the answer, with not one rebuttal. Unfortunately, this doesn't leave me too confident in the help I'm going to receive over there...
Doing some quick searches on reactivity of CaSO4 revealed that it can and does react with sodium bicarbonate (baking soda). I'm not convinced though that it's due to pH, it's likely linked to the chemical structure of Na2HCO3. What was the pH of the water when you measured it? Also, Plaster of Paris does not dissolve to this extent in water.
Current Species:
Camponotus fragilis
Novomessor cockerelli
Pogonomyrmex montanus
Pogonomyrmex rugosus
Manica bradleyi
What you say makes a lot of sense. The pH of the water, if I can remember right, I think was somewhere right around 7.








Gypsum crystals! Your hydrostone formicariums will start looking like this cave:

Oh duh, that shouldn't have been hard to figure out. Yeah it's obviously gypsum, and that's obviously what's dissolving.
So I just thought I would test the humidity in the hydrostone container after moving the colony out of it, and it actually came out to be 81% RH, one percentage point lower than the cement one, so even though it is not all soggy like the Hydrostone, the RH inside is just the same.
I've solved your corrosion problem.
Lose the sponge and stick the reservoir on top.
For additional reinforcement, add 5-10% cement to your Hydrostone mix, by weight.
It's a blue bottle with the head stuck in the plaster.
If you don't want to use a blue bottle, you can accomplish the same thing by welding two pieces of acrylic that form a 90 degree angle to two, perpendicular inside side walls of a container. Then, pour your Hydrostone to cover the gap at the bottom.
Edited by drtrmiller, February 1 2015 - 4:54 AM.
And how is that going to stop the plaster from dissolving?
It works because the resulting precipitate that would previously form on the sponge, and by its absence cause a pinhole, can no longer be displaced to the sponge to form a pinhole.
Edited by drtrmiller, February 1 2015 - 1:07 PM.
I'm pretty sure it's forming on the sponge, because it's dissolving into the water. The sponge in my pictures just had Hydrostone on the bottom of the tank. Anything you lay on Hydrostone that is wet, will dissolve the Hydrostone and leave a mark. If it's wet and touching the Hydrostone, it's going to dissolve it. I'm not sure how your bottle is getting water to the Hydrostone without touching it.
I'm pretty sure it's forming on the sponge, because it's dissolving into the water. The sponge in my pictures just had Hydrostone on the bottom of the tank. Anything you lay on Hydrostone that is wet, will dissolve the Hydrostone and leave a mark. If it's wet and touching the Hydrostone, it's going to dissolve it. I'm not sure how your bottle is getting water to the Hydrostone without touching it.
I have the same problem with plaster. When I water the nest it leaves a white residue on the walls.
 |
Ant Keeping →
General Ant Keeping →
Best Nest Material for Myrmecia?Started by AntInSpaceFilms , Apr 6 2025 |
|

|
|
Ant Keeping →
General Ant Keeping →
A Nest IdeaStarted by cooIboyJ , Mar 22 2025 |
|

|
||
Market Place →
General Market Place →
Trade: 3D-Printing Service for your Queens/Colonies (Los Angeles, CA)Started by JonathanH , Mar 4 2025 |
|

|
||
Market Place →
General Market Place →
Ender Ants: Naturalistic Formicariums & MerchandiseStarted by Ender Ants , Feb 7 2025 |
|

|
||
Ants & Myrmecology →
General →
Starting off a shopStarted by OwlThatLikesAnts , Feb 3 2025 |
|

|
0 members, 1 guests, 0 anonymous users